In-House Hospital Radiopharmacy
The ARRONAX in-house radiopharmacy is governed by the Nantes University Hospital. The radiopharmacy production premises house and operate both radionucleotide production and pharmaceutical manufacturing aspects in respect of radiation safety regulation and aseptic processing requirements.
The hot cell facility is subdivided into 2 categories, with 3 hot cells ffor radiochemistry and 5 hot cells for production of the radiopharmaceutical.
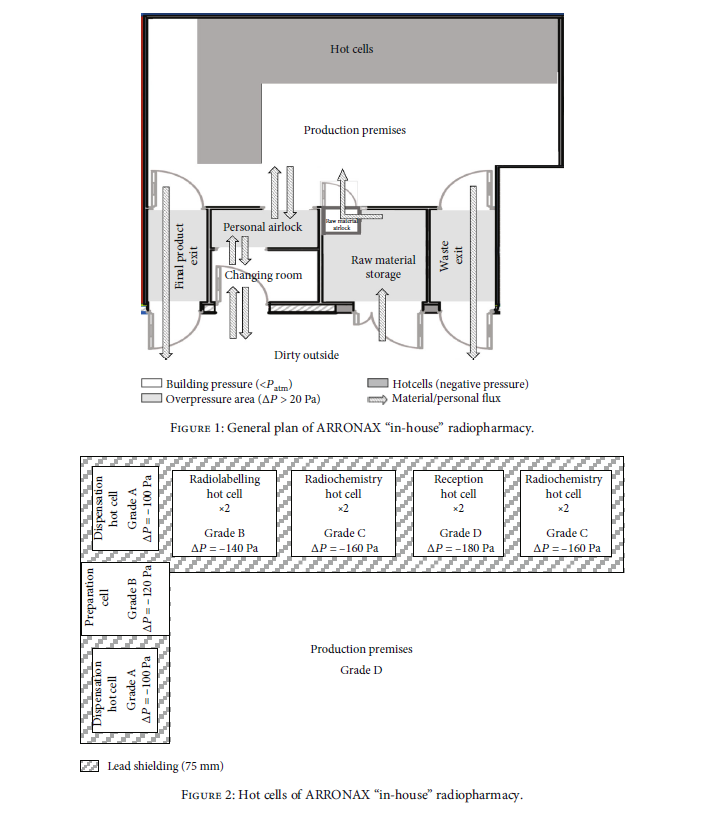
The ARRONAX in-house radiopharmacy also performs as a quality control laboratory (QC lab). In accordance with GMP requirements, the staff responsible for QC are independent of the production staff.
The design, commission, and operation of the ARRONAX in-house radiopharmacy comply with the bacteriological and environmental particulate requirements required for the production of sterile radiopharmaceuticals for use in early phase clinical trials in a hospital nuclear medicine department.
The ARRONAX in-house radiopharmacy is authorized by the local Regional Health Agency (ARS Pays de la Loire) for the preparation of radiopharmaceutical compounds dedicated to clinical trials in nuclear medicine, and by the French Nuclear Safety Authority (ASN) for the distribution of radiopharmaceuticals dedicated to clinical trials in nuclear medicine.
Our team is composed of :
- 2 Radiopharmacists
- 2 Radiochemist engineers
- Radiochemist technicians
Do not hesitate to contact for any questions.

